Unconventional Thinking for Exceptional Contract Manufacturing Value
Schivo is a vertically integrated, medical device contract manufacturer that applies unconventional thinking to achieve breakthroughs in cost, productivity, and time to market. We provide value that goes beyond manufacturing to optimize OEM profit and enhance the patient experience.
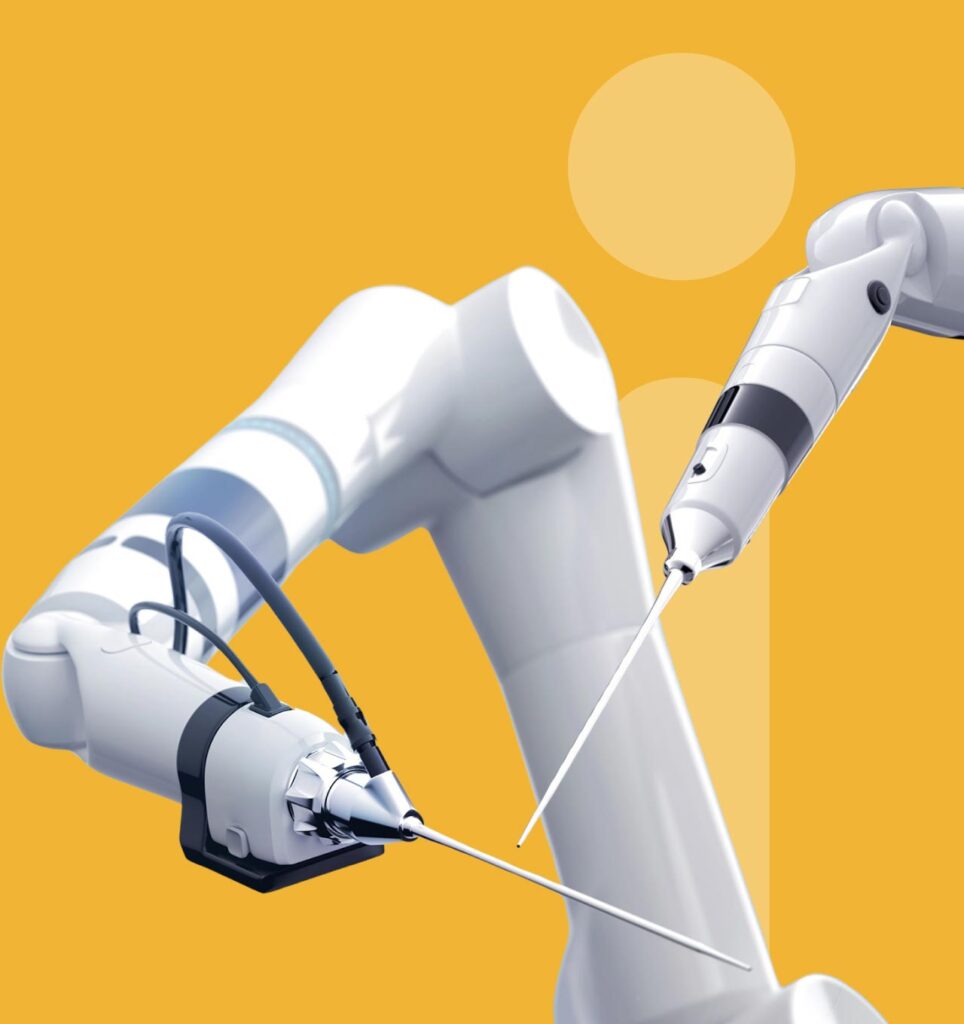
Manufacturing Solutions
Unbound by traditional approaches, our exceptional manufacturing expertise produces medical device components, sub-assemblies, and assemblies that offer dramatic breakthroughs in productivity, cost reduction, and risk mitigation. From new product introduction to ultra-high-quality production to packaging and logistics, Schivo’s vertical integration extracts cost and time across the value chain.
Our Expertise
Schivo is a trusted partner for global medical device companies producing minimally invasive and robotic-assisted surgical systems, cardiovascular and neurovascular devices, and orthopedic implants. We manufacture components and sub-assemblies for instruments, energy devices, and endo-mechanical products.

Minimally Invasive Surgery Solutions
Schivo’s unconventional thinking has created a wide range of minimally invasive surgery products that achieved dramatic cost and performance improvements.

Robotic-Assisted Surgery Solutions
Schivo’s ability to extract value from its best-practice global facilities has created robotic-assisted surgery components and sub-assemblies that maximize customer profit, reduce times to market, and improve the patient experience.

Life Science Solutions
Schivo’s AFP division specializes in manufacturing ultra-reliable fluid control components and sub-assemblies for chromatography laboratory instruments and genomic and clinical diagnostic systems.
Product Development
Our deep knowledge, unconventional approaches, and streamlined design process create innovative products with the fastest times to market, greatest OEM profit, and superior patient outcomes.
New Product Introduction
From concept to launch, Schivo’s proven NPI process goes beyond the limitations of customary and conventional product introduction methods. We work with OEMs early in the concept stage, linking design directly to manufacturing to ensure an efficiently designed and engineered product.
Qualification and Validation
Schivo tests and validates its processes with ISO-qualified methodologies prior to production. Then, each part‘s production data is recorded, tracked, and stored in the company’s manufacturing management platform for future reference and assurance it meets or exceeds specifications.
Regulatory File Submissions Support
Schivo fully supports the medical device OEM’s requirements for regulatory file submissions. Our company is registered with the U.S Food and Drug Administration (FDA) and complies with or exceeds their quality system requirements.
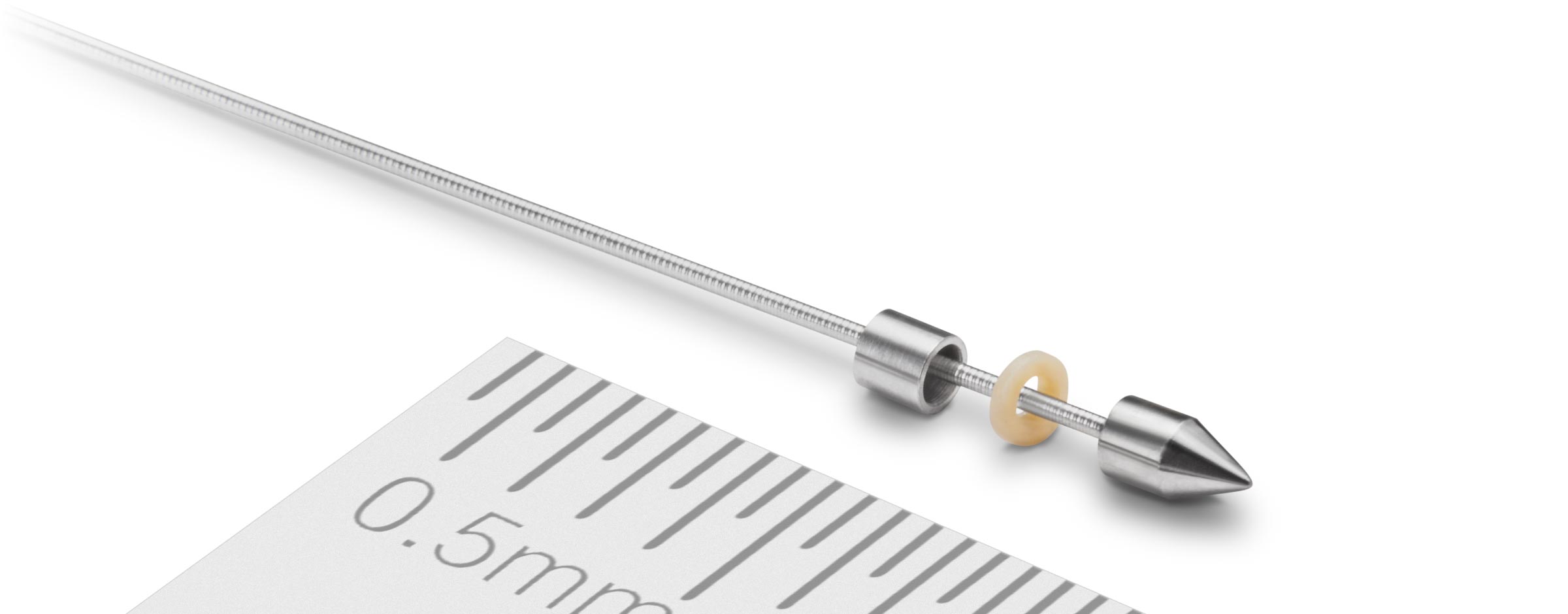
Our Products
Schivo applies its unconventional thinking to produce a wide range of high-quality components, complex sub-assemblies, and final assemblies. This approach generates value that goes beyond manufacturing to include a financial structure that ensures a competitive OEM product plus assures patients receive an effective procedure that insurers will reimburse.


About Schivo
Schivo is a global contract manufacturer of ultra-high-quality components, sub-assemblies, and assemblies for minimally invasive and robotic-assisted surgical systems, cardiology and neurovascular devices, and orthopedic implants.